
For the undiscovered eighth-row elements, mixing of configurations is expected to be very important, and sometimes the result can no longer be well-described by a single configuration. Similarity of valence shell electron configuration implies that we can determine the electron configuration of an atom solely by its position on the periodic. How many electron configurations does a chlorine atom have. Nitrogen can lose all 5 electrons resulting in a 5 charge. Chlorine has an electron configuration of 1s2 2s2 2p6 3s2 3p5. Now, let’s find phosphorus on the periodic table. Writing Electron Configurations Using Only The Periodic Table Youtube So Oxygens electron configuration would be O 1s22s22p4. So, in the case of Silicon, we have its electron configuration is Ne 3s2 3p2 which you can learn for. This process is integral for all types of chemical elements in chemistry and displays some significant features of the element. If we count the electrons in each orbital for Carbon’s configuration, we get 2+2+2 6 Phosphorus. The electron configuration is the process in which the chemical element distributes its electron into its orbitals. We can also reaffirm this answer by noticing that carbon is number six on the periodic table, and therefore has six electrons. In many cases, multiple configurations are within a small range of energies and the irregularities shown below do not necessarily have a clear relation to chemical behaviour. The electron shell configuration is 1s 2 2s 2 2p 2. The periodic table is a tabular display of the chemical elements organized on the basis of their atomic numbers, electron configurations, and chemical. Note that these electron configurations are given for neutral atoms in the gas phase, which are not the same as the electron configurations for the same atoms in chemical environments. As you go across a period from left to right, the nuclear charge increases so the valence level electrons are attracted more strongly to the nucleus, decreasing.
However there are numerous exceptions for example the lightest exception is chromium, which would be predicted to have the configuration 1s 2 2s 2 2p 6 3s 2 3p 6 3d 4 4s 2, written as 3d 4 4s 2, but whose actual configuration given in the table below is 3d 5 4s 1.
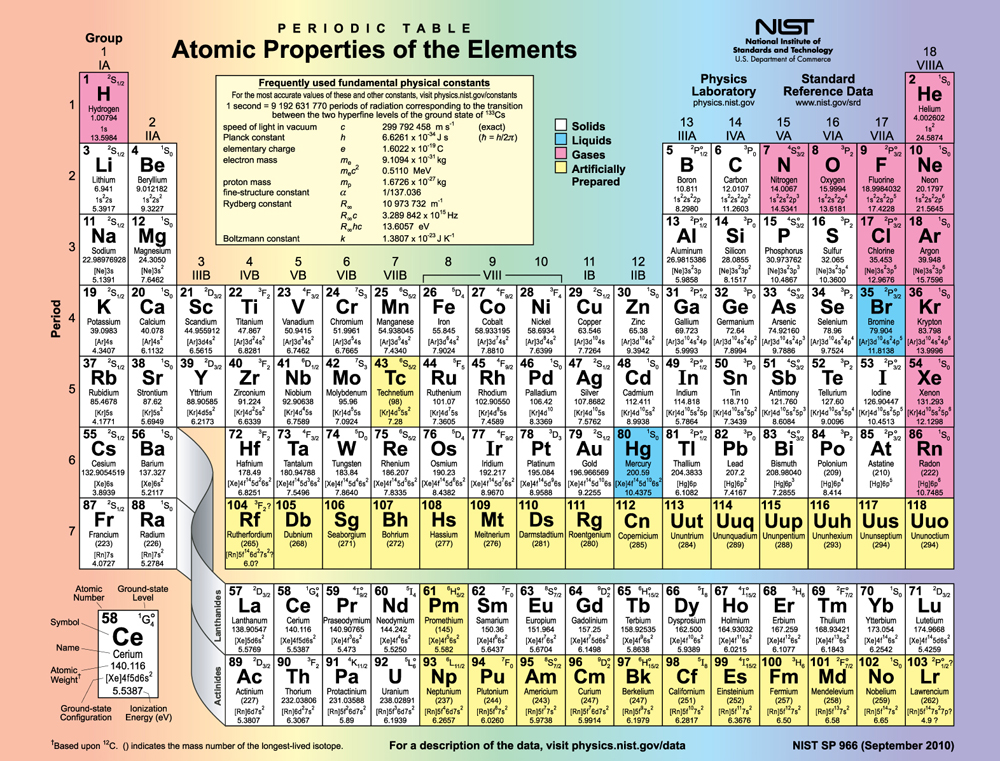
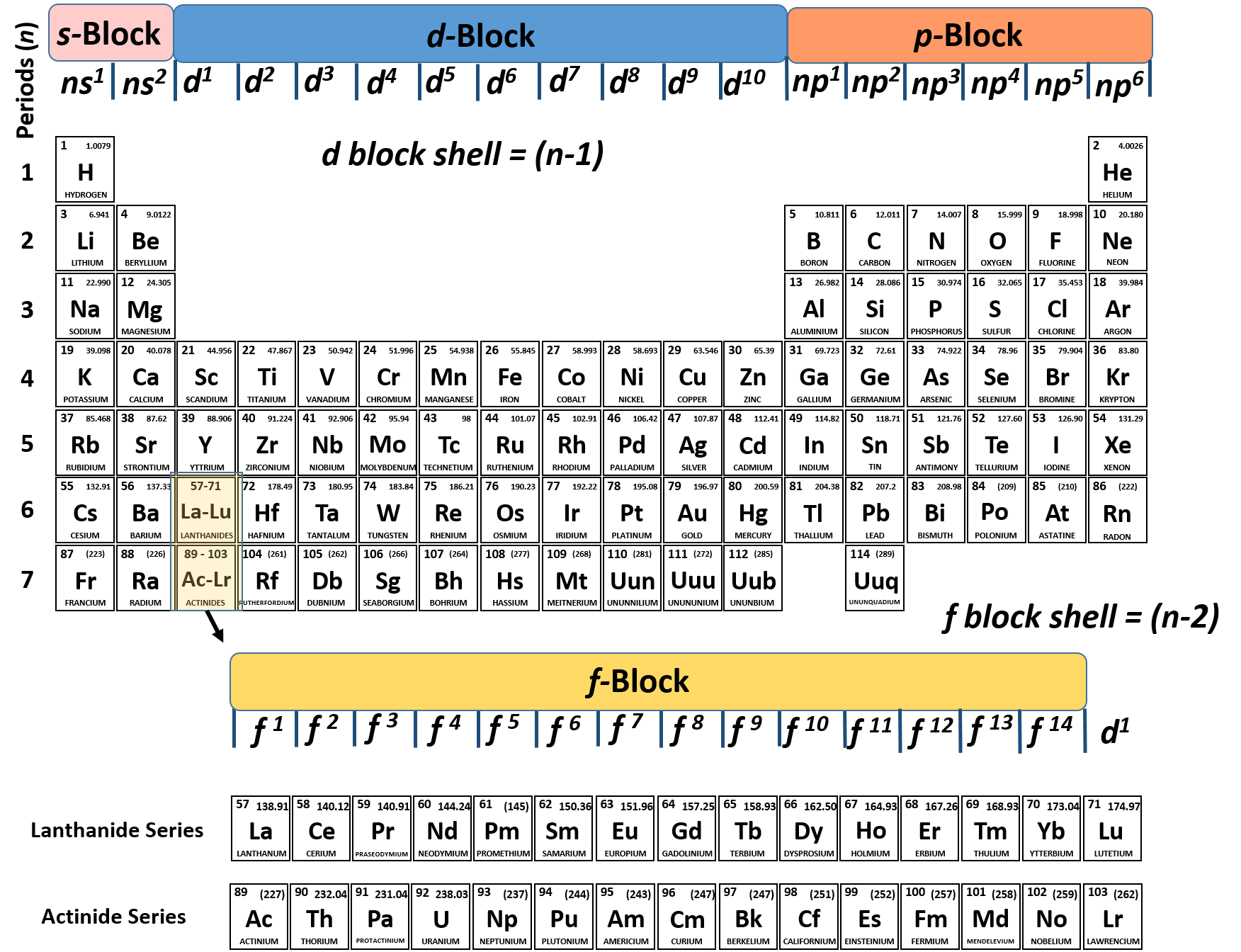
Electron configurations can be predicted by the position of an atom. Electron configurations of elements beyond hassium (element 108) have never been measured predictions are used below.Īs an approximate rule, electron configurations are given by the Aufbau principle and the Madelung rule. The arrangement of electrons in atoms is responsible for the shape of the periodic table. In Electronic Configuration electrons are arranged in various shells, Subshell and Orbital by following certain rules. For each atom the subshells are given first in concise form, then with all subshells written out, followed by the number of electrons per shell. Electron Configuration -The Electron Configuration of an Element Describes how Electrons are Distributed in their Atomic Orbitals. This page shows the electron configurations of the neutral gaseous atoms in their ground states.


 0 kommentar(er)
0 kommentar(er)
